Differentiation and adaptation among fishers in Western Canada: Evidence for validity of Pekania pennanti columbiana subspecies
1 Artemis Wildlife Consultants, Duncan, British Columbia, Canada
RW: https://orcid.org/0000-0002-5381-8514
Weir, R.D. (2025). Differentiation and adaptation among fishers in Western Canada: Evidence for validity of Pekania pennanti columbiana subspecies. Stacks Journal: 25002. https://doi.org/10.60102/stacks-25002

Abstract photo. Drawing of a female fisher by Andrea Toth.
Abstract
Recent work has shown that fishers (Pekania pennanti) in Western Canada are split into at least 2 populations, with the endangered Columbian population that occurs in central British Columbia being genetically distinct from fishers in the Boreal population of northern and northeastern portions of the province. To better understand other differences that may exist between these populations, I examined morphological, diet, and age-structure differences using data collected from 329 carcasses collected from trappers between 1989-1993 and live-captures of 105 fishers by researchers between 1996 and 2023. Fishers from the Columbian population had significantly different axial and appendicular skeletons from those in the Boreal population and were significantly smaller in body mass, body and tail length, foot area, neck girth, and chest girth. Despite their smaller overall body mass, fishers in the Columbian population had significantly higher foot-loading than did fishers from the Boreal population. Fishers in the Columbian population also had significantly different winter diets than those in the Boreal population, ingesting mid-sized mammals, grouse, and squirrels more often and ungulates and small mammals less frequently than did fishers in the boreal forest. Age structures were not significantly different between populations but were dominated by juvenile and subadult individuals. These significant differences in morphology and diet were coincident with numerous significant differences in the biotic and abiotic environments in which each population occurred and, when coupled with the genetic and possible biogeographical history of the 2 populations, suggested considerable adaptive differentiation that substantiates the subspecies concept for P. p. columbiana.
Keywords: fishers, Pekania pennanti, subspecies, morphometry, biogeography, age structure, diet, British Columbia
Introduction
Fishers (Pekania pennanti) are a medium-sized forest-dependent predator in the mustelid family that broadly occur across the Northern Forest, Northwestern Forested Mountains, and Eastern Temperate Forest ecological regions of North America. Early taxonomists proposed 3 subspecies of fishers based largely on skull and pelage characteristics (Rhoads 1898, Goldman 1935, Grinnell, Dixon, and Linsdale 1937): P.p. pennanti, which is widespread, common, and occurs east of the continental divide in Canada and the northeastern USA; P.p. pacifica, which is rare and occurs along coastal ranges in Oregon and California; and P.p. columbiana, which occurs in central British Columbia, Idaho, and Montana. However, a later assessment of several hundred pelts and skulls collected from throughout the species’ range found that differences in measurements were so slight that the nature of variation could not be determined (Hagmeier 1959). As a result, the subspecies concept was largely considered to be invalid (Powell 1993) and the species has since been considered to be monotypic.
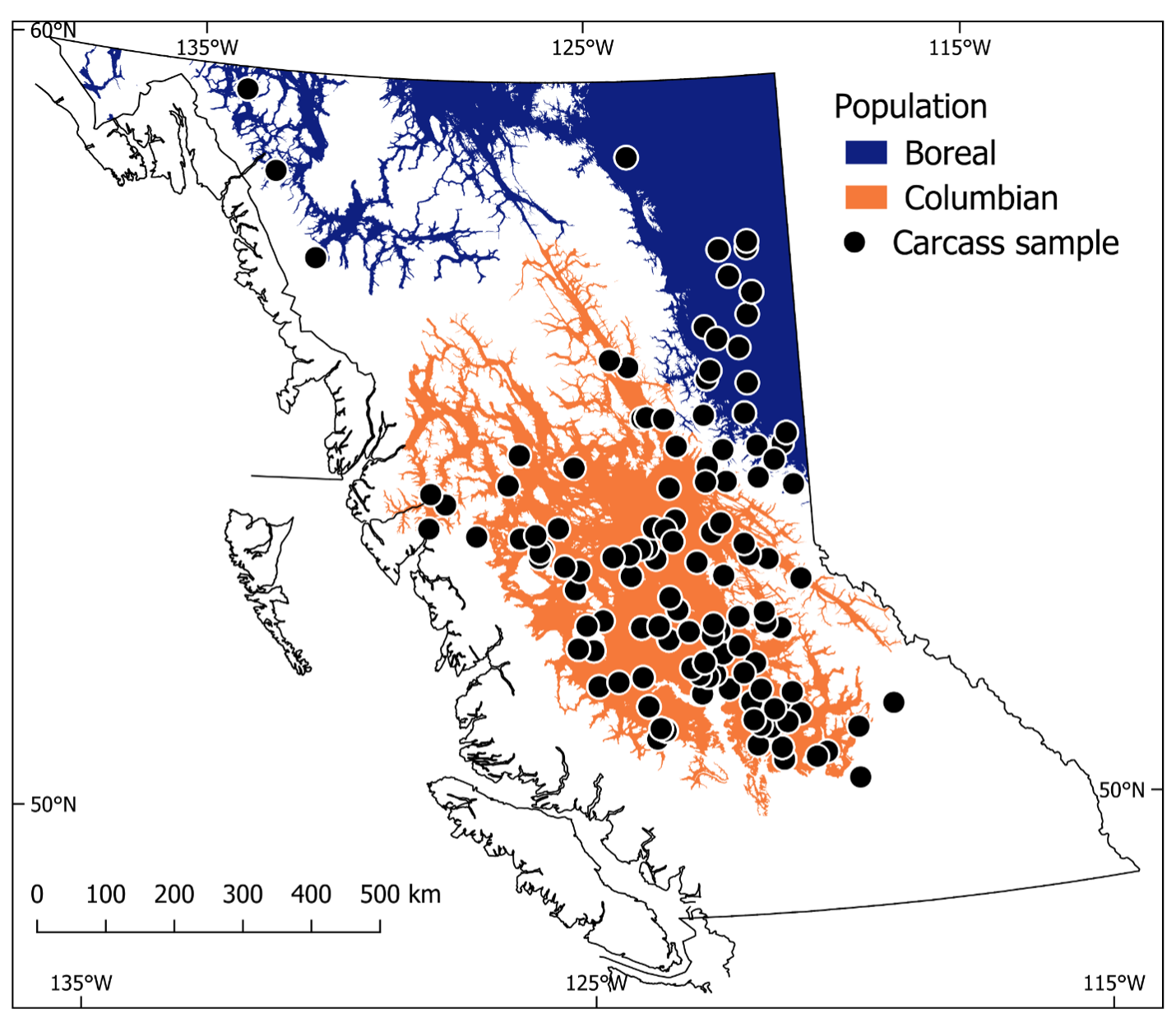
Genetic evidence, however, suggests that the subspecies concept for fishers may be legitimate after all (e.g., Drew et al. 2003, Knaus et al. 2011). Notably, recent population genetic analyses showed that fishers in the westernmost Canadian province of British Columbia occur as 2 populations (Weir et al. 2024) that are spatially consistent with the distribution of P.p. columbiana and the western edge of P.p. pennanti. The Columbian population occurs in the central portion of the province to the west and south of the Rocky, Omineca, and Skeena Mountain ranges, whereas the Boreal population occurs to the north and east of these ranges (Figure 1), with little genetic exchange through this geographic barrier. Genetic characteristics of these populations (i.e., level of differentiation among nuclear and mitochondrial genes; Weir et al. 2024) suggest that the Columbian population may have been geographically separated for a considerable length of time from fishers in the Boreal population.
The biotic and abiotic environments in which the Columbian population occurs are substantially different from those found in the Boreal population. The Columbian population occurs predominantly in the Sub-Boreal Spruce, Sub-Boreal Pine-Spruce, and Interior Douglas-Fir biogeoclimatic zones, whereas the Boreal population occurs almost exclusively in the Boreal Black and White Spruce biogeoclimatic zone. The biogeoclimatic zones that support the 2 populations differ substantially in climate, landforms, and climax vegetation communities (Pojar, Klinka, and Meidinger 1987) and produce ecosystems that vary markedly in tree species composition, forest productivity and dynamics, structural diversity, and animal assemblages (MacKinnon, Meidinger, and Klinka 1992, MacKenzie and Meidinger 2018).
These differences in biotic and abiotic environment may have been sufficient, when coupled with periods of isolation, for natural selection and adaptation to have resulted in differences in morphology, behaviour, and habitat exploitation. Specifically, local adaptations to the unique environmental conditions found in the central interior of British Columbia may have resulted in measurable differences in morphology, behaviour, diet, and possibly age structure of fishers in the Columbian population from their eastern counterparts.
The objective of this study was to further evaluate the extent to which fishers in the Columbian population had differentiated from fishers in the Boreal population of British Columbia beyond population genetics differences identified by Weir et al. (2024). Specifically, I wanted to assess variation in morphometry, diet, reproductive rates, and age composition to better understand the differences between the populations, document possible adaptations to the local environments, and assess the subspecies concept for this species. Given the scope and scale of genetic distinctiveness, differences in biotic and abiotic environments, and possible biogeographical histories, I predict that fishers in the Columbian population diverged from the nearest neighbouring population in the boreal forest in terms of body morphometry and diet such that this population may be described as a valid subspecies. This information will be exceptionally valuable not only to understand at-risk fisher populations in British Columbia, but also to provide better information for decision-makers upon which to evaluate the status and identify conservation or management actions needed for each population.
Methods and Materials
I used 2 sources of data to assess differences in morphology among fishers in British Columbia: skinned carcasses of dead animals trapped by licensed fur-trappers and measurements of live animals collected during handling for radiotelemetry studies. These data were not directly comparable because the condition of the animals in each were substantially different; carcasses had their skins removed whereas live animals were fully intact, which affected body mass, skull and girth measurements. As such, comparisons between populations were only made within each data set.
Carcass collection and sampling
I collected fisher carcasses from throughout British Columbia that were legally trapped during open trapping seasons by licensed fur-trappers between 1989 and 1993. All carcasses were skinned by the trapper, bagged, labeled with the registered trapline from which the animal was trapped, frozen and transported to Ministry of Environment facilities in Williams Lake, British Columbia for processing. British Columbia uses a registered trapline system, which is an area-based tenure whereby a registered trapline owner has the exclusive right to harvest pelts from furbearing animals, so the provenance of each carcass was relatively high.
I thawed, measured and sampled each carcass within 15 months of submission by the trapper. I recorded the sex of the individual and weighed each intact carcass (i.e., those not missing body parts other than skin) to the nearest 100 g using a spring scale. I then measured the length of the body (i.e., nose tip to sacrum), tail (i.e., sacrum to last vertebrae), and chest girth. I measured the length from the shoulder joint to the ankle and the ventral surface of the chest to ankle for the forelimbs with elbow joint held at 135° and the hip joint to ankle and ventral surface of the abdomen to ankle for the hindlimbs with knee joint held at 135°. I visually assessed the relative amount of subcutaneous fat on each carcass, using the following categories: none (no fat), low (fat limited to minor deposits in the inguinal region), medium (fat deposits in the inguinal and shoulder regions), and high (copious fat deposits in the inguinal, shoulder, and other regions). For males, I measured the length of the baculum and testes width and length. I also measured the width of the zygomatic arch, distance from the bottom of the mandible to the peak of the frontal process, and length from the premaxilla to the interparietal process of each skull.
Because trappers leave the feet and lower leg furred when skinning fishers, I was able to assess foot-loading by measuring several aspects of the unskinned feet of each carcass. I measured the distance between the medial edge of toe 1 and lateral edge of toe 5 as the foot width and the anterior edge of toe 4 and the posterior edge of interdigital pad 4 as the foot length (see Zielinski and Truex 1995, Fig. 1 for reference points). I estimated the total area of one front and one hind foot using the following formula for each measured foot:
I estimated the total foot area for each individual by summing 2 × front foot area and 2 × hind foot area. I then calculated the minimum foot-loading for each individual as if it was standing on all 4 feet by dividing the body mass of the skinned carcass by the total foot area. During winter, fishers tend to travel using a galloping gait pattern that loads 3 feet simultaneously (Raine 1983), so I also estimated the minimum foot-loading for a moving fisher by dividing body mass by the sum of the foot area of the front 2 feet and 1 rear foot.
I collected several samples for further analysis from each carcass. I removed an upper premolar (PM4) for cementum ageing (sensu Poole et al. 1994) and the stomachs for identification of contents (Weir, Harestad, and Wright 2005). I also removed the uterus and ovaries of females to tally corpora lutea following the methods of Douglas and Strickland (1987). Teeth were aged by Matson’s Laboratory (Milltown, Montana, USA) and the corpora lutea counts were completed by serially sectioning each ovary and staining with Masson’s trichrome following methods of Strickland (1982). Although confidence in cementum ageing varied among tooth samples, I assumed that this variation occurred as chance error and did not represent bias in the actual age structure. I classified fishers as juveniles (<12 mo), subadults (<2 yr), or adults (≥2 yr) based on tooth cementum annuli estimates.
Live animal capture, handling, and sampling
Live fishers were captured, immobilized, measured, and radio-tagged as part of 5 research projects involving fishers from both the Columbian (Weir and Corbould 2008, Davis 2009, S. Frey, Ministry of Water, Land and Resource Stewardship, unpublished data) and Boreal (Weir et al. 2013, Simpson et al. 2013) populations of fishers. Each fisher was sexed based on physical examination of external genitalia and weighed with a spring balance once immobilized. I assigned age class using cementum annuli in cases where a premolar 1 tooth was extracted from live fishers; otherwise, I classified fishers as adults if sagittal crests were noticeably evident by palpation, if chest girth was ≥23 cm for males, or if nipples were swollen enough to be easily visible for females. Non-adults were assigned to juvenile age class if body mass was <1.7 kg for females or <3.7 kg for males and tooth wear was minimal; all others were assigned to subadult age class. This classification scheme was based upon the 75th percentile of observations for these attributes among individuals of known age. A subset of the carcass morphological measurements was collected on live-captured fishers, including body mass, zygomatic arch breadth, chest girth, body length, and tail length. I also measured neck girth for 2 sets of the live-captured animals (Weir and Corbould 2008, Weir et al. 2013). All capture, handling, and radio‐tagging methods met or exceeded capture and handling guidelines outlined in the protocols for Wildlife Capture and Handling (Resource Inventory Committee 1998), were approved by a member of the Canadian Council on Animal Care, and were carried out under Wildlife Act permits C076979, WL05‐19549, FSJ05‐9483, and FJ10‐67379.
Morphometric, diet, and age structure comparisons
I assessed differences in morphometry between fishers from the Columbian population and those from the Boreal population using data from both carcasses and live animals, analyzed as 2 separate data sets. Aspects of fisher morphometry vary with sex and age class (Douglas and Strickland 1987). As such, I used the adonis2 function of the vegan package (Oksanen et al. 2024) to conduct a non-parametric multivariate analysis of covariance (i.e., MANCOVA) to examine differences in morphometric measurements between populations, whereby I was able to control for the effects of sex and age class by including these categorical variables as covariates in the model. I accounted for unequal sample sizes among groups by conducting 1000 bootstrap samples and avoided multicollinearity by excluding highly correlated variables (i.e., |r| >0.80; e.g., Tabachnick and Fidell 2019) in the multivariate models. In the case where a significant difference occurred between populations, I conducted post-hoc nonparametric univariate analyses of variance (i.e., Wilcox test), controlling for sex and age class by using residual analysis, to identify which morphometric features were significantly different between populations.
I also used the stomach contents data from Weir, Harestad, and Wright (2005) to compare and contrast the winter diet of fishers collected from the Columbian population to those from the Boreal population. I conducted a chi-square goodness of fit test to evaluate the frequency of occurrence of prey species in each stomach among the 2 populations using 5 categories of prey: small mammals (i.e., mice, voles, shrews), galliform birds, squirrels (i.e., red squirrels Tamiasciurus hudsonicus, northern flying squirrels Glaucomys sabrinus), medium-sized mammals (i.e., snowshoe hare Lepus americanus, North American beaver Castor canadensis, porcupine Erethizon dorsatum), and ungulates (deer Odocoileus spp., moose Alces alces, domestic cattle Bos spp.). I identified significant differences in the proportion of occurrence for each prey group between populations using Bonferroni-adjusted Z-tests. I set the acceptable Type I error rate for all analyses at α = 0.05.
I used cementum annuli ages for fishers from both the carcass and live-capture collections to provide descriptive statistics for the age and sex structure of each population.
Forest structure comparisons
I evaluated differences in forest structure between each population of fishers using data collected in the 2 biogeoclimatic zones that dominated the range of each population: the Sub-Boreal Spruce (SBS; Columbian population) and Boreal White and Black Spruce (BWBS: Boreal population) biogeoclimatic zones. Briefly, the biogeoclimatic ecosystem classification system is founded on the observation that, within broad areas of homogenous climate and geology called biogeoclimatic zones, consistent ecosystems form on sites depending upon the soil moisture and soil nutrient regime found there (Pojar, Klinka, and Meidinger 1987, MacKinnon, Meidinger, and Klinka 1992). These ecosystems have plant assemblages and structural attributes that change in predictable patterns as succession proceeds following disturbance.
I measured structural attributes in each relatively homogenous vegetation community formed by the intersection of soil moisture, soil nutrient, and structural stage in each biogeoclimatic zone. I collected vegetation plot data using identical standardized methods at random geocoordinates among 6 structural stages (ranging from herb to old forest; British Columbia Ministry of Forests and Range and British Columbia Ministry of Environment 2010:23) of sites comprised of combinations of soil moisture and nutrient regime for each biogeoclimatic zone (see Weir and Corbould 2008 and Weir, Phinney, and Lofroth 2012 for sampling methodology and structural calculations). In total, I measured 8 vegetation cover, 9 coarse woody debris (CWD), and 7 tree density variables at each sampling site (see Table 4 for descriptions).
I used the adonis2 function of the vegan package (Oksanen et al. 2024) to conduct a non-parametric MANCOVA to evaluate if ecosystems (as dictated by the intersection of soil moisture, soil nutrient, and structural stage) in each biogeoclimatic zone were structurally different. I controlled the effects of soil moisture, soil nutrient, and structural stage by including these as covariates in the model and conducted 1000 bootstrap samples to account for sampling imbalances. This approach allowed for the equitable comparison of structural attributes among equivalent stands in each biogeoclimatic zone (e.g., a young forest, mesic moisture, and medium nutrient stand in the BWBS zone compared to a young forest, mesic moisture and medium nutrient stand in the SBS zone). Preliminary analysis of the data identified several pairs of correlated structural variables (i.e., |r|≥0.6) such that I included only the one that had previously been most closely linked to habitat use by fishers (e.g., Weir and Harestad 2003), which left 16 structural variables in the MANCOVA analysis. In the case where significant differences occurred, I conducted post-hoc nonparametric univariate analyses of variance (i.e., Wilcox test) controlling for soil moisture, soil nutrient, and structural stage by using residual analysis to identify which structural features differed between biogeoclimatic zones.
Results
I compiled morphometric and age data on 434 fishers from both populations of fishers in British Columbia. I collected carcasses of 329 fishers (190 F, 139 M; 236 from Columbian population, 45 from Boreal population, and 53 for which location data was not recorded) that were trapped from 137 registered traplines (Weir 1995) that covered approximately 220,000 km² (Figure 1) and used 281 of these carcasses for analysis. The median year of birth of fishers in the carcass sample, as estimated from cementum annuli, was 1990 (SD = 2 years, n = 277). I also compiled morphometric data from 105 fishers that were live-captured and radio-tagged as part of 5 radio-telemetry research projects: Williston region between 18 November 1996 and 21 March 2000 (14 F, 7 M; Columbian population; Weir and Corbould 2008), Chilcotin region between 16 December 2005 and 1 February 2008 (15 F, 10 M; Columbian population; Davis 2009), Kiskatinaw region between 5 March 2005 and 16 March 2008 (17 F, 9 M; Boreal population; Weir et al. 2013), Peace River region between 12 January 2011 and 15 March 2013 (10 F, 6M; Boreal population; Simpson et al. 2013), and 100 Mile House region between 3 January and 27 November 2023 (11 F, 6 M; Columbian population; S. Frey, Ministry of Water, Land and Resource Stewardship, unpublished data). The median year of birth of fishers among these 5 research projects was estimated to be 2005 (SD = 8 years, n = 96). I also used age and sex information from 149 fishers that were captured in the Columbian population and translocated to Washington State, USA between 2008 and 2017 (Lewis 2014, Lewis et al. 2022) to augment the characterization of age structure for this population.
Morphometry
When controlled for sex- and age-effects, fishers in the Columbian population had significantly different morphometries from those in the Boreal population of British Columbia in both the carcass sample (explained variance R² = 0.0190, p < 0.001) and the live-capture sample (R² = 0.039, p < 0.001). Sex (carcasses: R² = 0.5029, p < 0.001; live-captures: R² = 0.572, p < 0.001) and age class (carcasses: R² = 0.0113, p < 0.013; live-captures: R² = 0.042, p < 0.002) were significant explanatory covariates in the multivariate analysis.
Both carcass and live-caught fishers from the Columbian population were significantly lighter than fishers from the Boreal population (carcass: W = 3765, p <0.005; Table 1; live-captures: W = 725, p <0.001, Table 2). Both fisher carcasses and live-captured fishers from the Columbian population had significantly shorter body length (carcasses: W = 396, p <0.001, Table 1; live-captures: W = 640, p <0.001, Table 2) and tail length (carcasses: W = 2512, p <0.001, Table 1; live-captures: W = 749, p <0.001, Table 2) than did those from the Boreal population. Chest girth was significantly smaller among fishers live-captured in the Columbian than the Boreal populations (W = 972, p <0.05; Table 2), but not for carcasses (W = 3095, p <0.217). Live-captured fishers from the Columbian population also had significantly smaller neck girths than did those from the Boreal population (W = 449, p <0.013, Table 2). Fisher carcasses from the Columbian population had significantly shorter distances between their chest and ankle than fishers from the Boreal population (W = 1788, p <0.002), but were not significantly different with respect to shoulder-ankle (W = 3164, p <0.919), hip-ankle (W = 2672, p <0.427), or abdomen-ankle distances (W = 1594, p <0.058).

Table 1. Morphometry of 281 skinned male and female fisher carcasses collected from throughout the Columbian and Boreal populations in British Columbia, Canada, 1989-1993. Features that were significantly different between populations, when controlled for sex and age class, appear in bold. Values are shown as x̄ (SD, n). Corpora lutea counts are for adult female fishers only. See Supplemental Information SI1 for values for each population, sex, and age class.
I did not detect differences in skull morphometrics, when controlled for sex- and age-effects, between the 2 populations. The width of the zygomatic arch, head depth, and head length were not significantly different between fisher carcasses collected in the Columbian and Boreal populations (width of zygomatic arch: W = 3105, p <0.082; head depth: W = 3749, p <0.648; head length: W = 3515, p <0.390; Table 1). The width of the zygomatic arch among live-captured fishers was also not significantly different between the Columbian and Boreal populations (W = 183, p <0.252; Table 2). Interestingly, the ratio between neck girth and width of the zygomatic arch was significantly lower among fishers from the Columbian population than the Boreal population (W = 83, p <0.011, Table2).
I also detected substantial differences between the 2 populations in total foot area and foot-loading. When controlled for sex and age-class, the total foot area of fisher carcasses from the Columbian population was significantly smaller than for fishers from the Boreal population (W = 390, p <0.001, Table 1). Despite their smaller overall body mass, fisher carcasses in the Columbian population had significantly higher foot-loading than did fishers from the Boreal population when standing on all four feet (W = 1021, p <0.005) or using a galloping gait (W = 1004, p <0.008).

Table 2. Morphometry of 105 live-captured fishers from the Columbian and Boreal populations in British Columbia, Canada, 1996-2023. Features that were significantly different between populations, when controlled for sex and age class, appear in bold. Values are shown as x̄ (SD, n). See Supplemental Information SI2 for values for each population, sex, and age class.
Diet
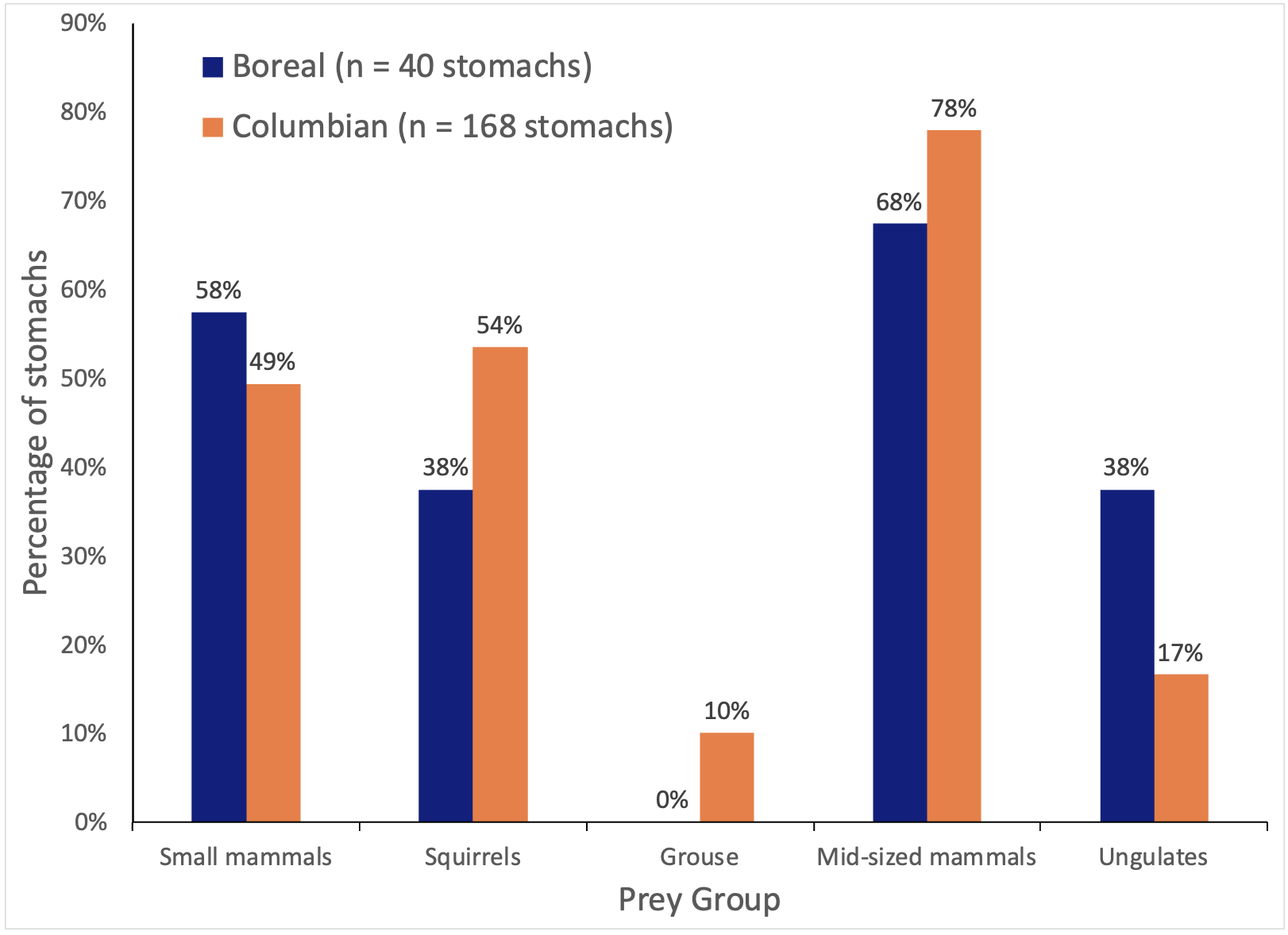
I evaluated stomach contents of 208 fishers killed by trappers in British Columbia between 1989 and 1993 using the data collected by Weir, Harestad, and Wright (2005), which included 21 different species of prey ranging in size from shrews (Sorex spp.) to ungulates (e.g., moose). On average, fishers in the Columbian population had 2.17 species of prey in their stomach (SD = 1.74, n = 168), whereas fishers in the Boreal population had 2.05 species per stomach (SD = 1.40, n = 40).
Diets were significantly different between fishers in the Columbian and Boreal populations (χ² = 13.589, df = 4, p < 0.009). Columbian fishers consumed mid-sized mammals (i.e., snowshoe hares, beavers, porcupines), grouse, and squirrels more frequently and ungulates and small mammals less frequently than did fishers in the Boreal population (Bonferroni-adjusted Z-tests, p ≤ 0.05; Figure 2). I did not detect any difference in fat scores of carcasses between populations (χ² = 0.972, df = 3, p < 0.8), with 89% (199 of 223) of carcasses from the Columbian population and 86% (37 of 43) of carcasses from the Boreal population having visible fat deposits.
Age structure and reproduction
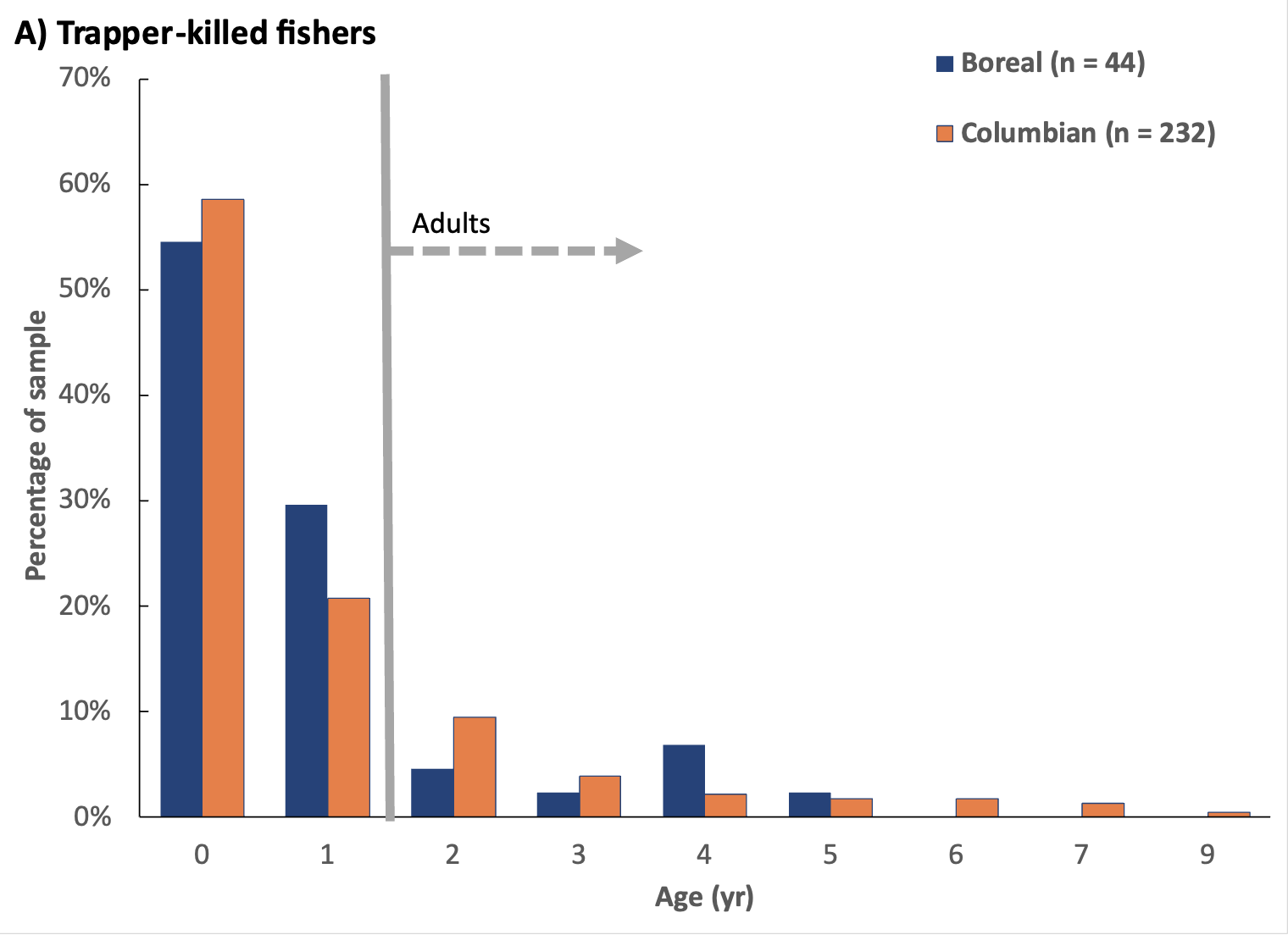
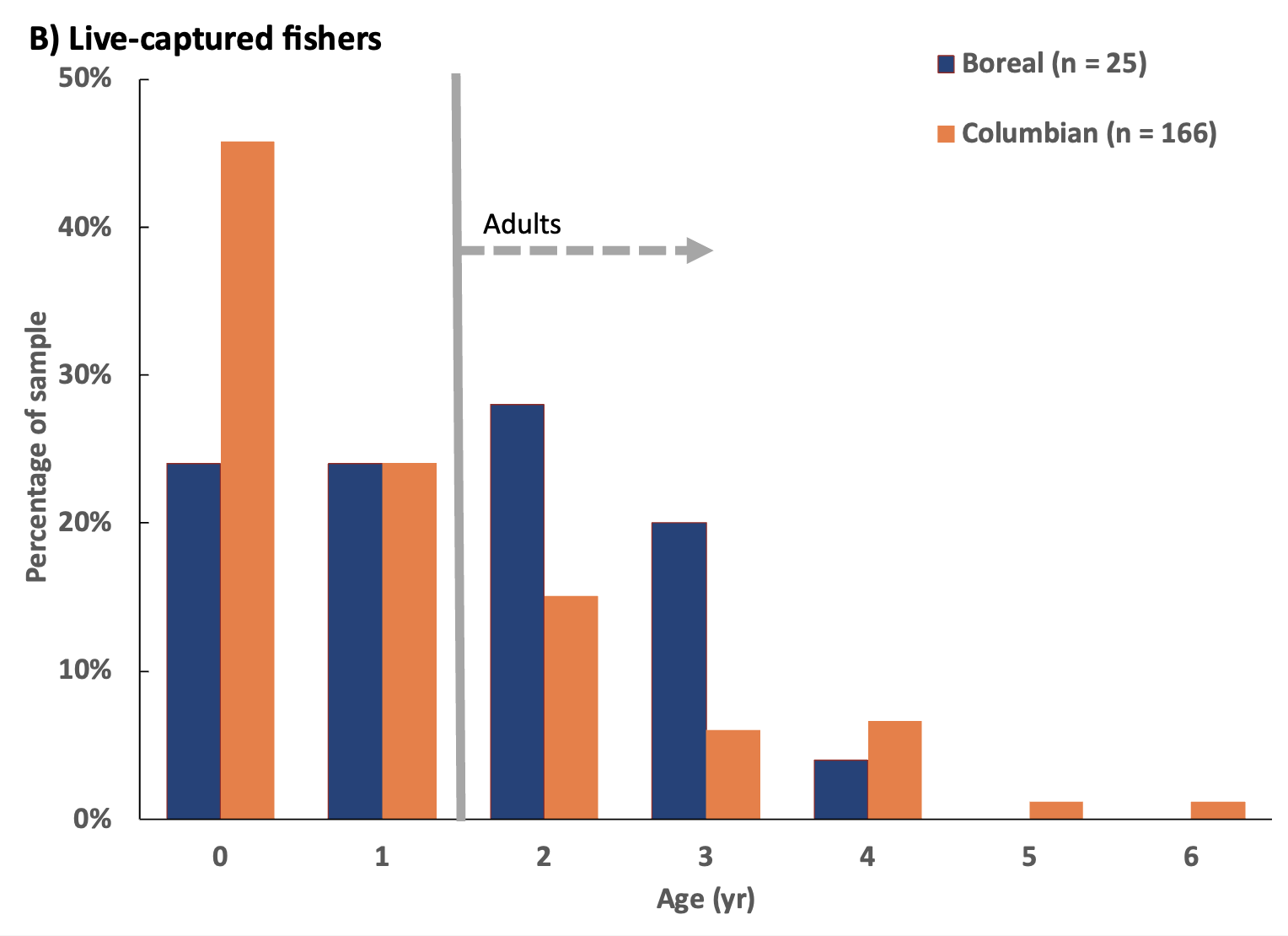
I collected cementum annuli ages from 276 carcasses and either cementum annuli (191) or age estimates (54) for 245 live-caught fishers (Figure 3). Among the carcass sample, 160 (58%) were juvenile fishers (i.e., <1 yr), 61 (22%) were subadults (i.e., 1-2 yr), and 55 (20%) were adults (i.e., ≥ 2 yr). I did not detect a significant difference in the distribution of age classes among fisher carcasses collected from the Columbian and Boreal populations (χ² = 1.843, df = 2, p < 0.4). Among live-caught fishers in both populations, 92 (38%) were juveniles, 55 (22%) were subadults, and 98 (40%) were adults. Within the Columbian population, 46% of live-captured fishers were juvenile at the time of first capture (76 of 166; 34F, 42M), 24% were subadult (40; 24F, 16M), and 30% were adult (50 of 166; 34F, 16M). Although juvenile fishers formed a larger proportion of the live-captured sample in the Columbian population (i.e., 46% compared to 24% in the Boreal population) and adults were a larger proportion of the Boreal population (i.e., 52% compared to 30% of Columbian population), age structures among the live-caught sample were not significantly different between the 2 populations (age class: χ² = 4.854, df = 2, p < 0.088; cementum annuli ages: χ² = 10.818, df = 6, p < 0.094). Among both carcass and live-captured fishers, the average age of adults (i.e., individuals ≥2 yr) in the Columbian population was 3.17 yr (SD = 1.51, n = 98), whereas it was 2.85 yr (SD = 0.93, n = 20) in the Boreal population.
Although age distribution was not significantly different among sexes (χ² = 7.20, df = 5, p ≤ 0.206), the ratio of females to males among all fishers sampled within the Columbian population varied from near 1:1 for juveniles (i.e., age 0 yr), to about 5:3 for 1 year-old fishers, and near 2:1 for adults (Table 3). Within the Boreal population, a similar trend occurred where the ratio of females to males was near 5:3 as juveniles but increased to about 2:1 for 1 year-olds and adults (Table 3), although age distribution for this population was also not significantly different among sexes (χ² = 1.17, df = 2, p ≤ 0.557).
I did not detect a significant difference in the number of corpora lutea in the ovaries of adult female fishers between the Columbian and Boreal populations (t = 0.14, p ≤ 0.89, Table 1), although sample sizes were very low from the Boreal population (n = 12). Overall, 22% (13 of 60) of adult females in the Columbian population had no corpora lutea present, whereas 3 of 12 adult females (25%) in the Boreal population had no corpora lutea present. There was a significant, weakly positive effect of age on corpora lutea count overall (F2,69 = 3.506, p < 0.035; R² = 0.09). I did not detect any difference in baculum size (F1,58 = 0.0295, p < 0.864), testes length (F1,67 = 1.903, p < 0.17), or testes width (F1,62 = 0.0006, p < 0.981) among male fisher carcasses collected from either population when controlled for age class (Table 1).

Table 4. Comparisons of forest structure observed at 287 vegetation plots among ecosystems of the Sub-Boreal Spruce biogeoclimatic zone (SBS; i.e., Columbian population) and Boreal White and Black Spruce biogeoclimatic zone (BWBS; i.e., Boreal population). Descriptive statistics are irrespective of soil moisture, soil nutrient, and structural stage upon which the multivariate analysis was based. See Weir and Corbould 2008 and Weir, Phinney, and Lofroth 2012 for detailed descriptions of structural attributes.
Forest structure comparisons
I conducted 287 vegetation plots among the 2 biogeoclimatic zones that dominated each population of fishers in British Columbia. Forest structure was significantly different between the SBS and BWBS biogeoclimatic zones, when controlled for soil moisture, soil nutrient, and structural stage (R² = 0.048, p < 0.001; Table 4). The cover of coniferous trees, coniferous shrubs, and shrubs >2m tall was higher in the SBS zone compared to the BWBS zone, although cover of deciduous trees was higher in the BWBS zone than the SBS zone. The distribution and abundance of CWD was also different between the 2 biogeoclimatic zones, with the total volume, density of pieces, and total length of elevated large pieces being significantly higher in the SBS zone than the BWBS zone. The aggregation index of CWD pieces, however, was significantly higher in the BWBS zone compared to the SBS zone, suggesting that CWD pieces were more highly clumped in the SBS compared to the BWBS zone. The density of trees >35 cm dbh was significantly higher in the SBS compared to the BWBS zone, whereas total density of stems >15 cm dbh and dead standing trees was higher in the BWBS zone than the SBS zone.
Discussion
Fishers from the Columbian population of central British Columbia showed consistent, significant differences in morphology and diet from their nearest counterparts in the Boreal population. Individuals from this population were reliably lighter, had more compact axial and appendicular skeletons, and had winter diets that focused more heavily on mid-sized prey than fishers from northeastern British Columbia. I hypothesise that this suite of differences are more than simple expressions of phenotypic plasticity and likely developed to deal with different local environments in both current-day distributions and Pleistocene refugia. Specifically, the lighter body mass, more compact axial skeleton, smaller feet, and different diet of fishers in the Columbian population may have developed or occurred as responses to climate, ecosystem, and prey communities that persist today in the central interior of British Columbia and confer advantages to these fishers. I further hypothesise that the divergence in body size, shape, and diet observed among fishers in British Columbia, when coupled with observed patterns of genetic divergence and differentiation, suggests that the Columbian and Boreal populations of fishers in British Columbia have substantially different biogeographical histories that, in conjunction with the effect of differing current-day environments, may have produced the differences observed today. These significant differences in morphology and diet coincided with numerous significant differences in the biotic and abiotic environments in which each population occurs and provide consistent support for these hypotheses.
Considerable structural differences existed between forests that support fishers in the Columbian and Boreal populations and these different environments may have exerted adaptive pressure that contributed to the different body shapes observed in this study. Although they occasionally capture prey and move through the forest canopy, fishers travel and forage primarily on the forest floor (Powell 1993). As a result, structural complexity of the forest floor likely plays an integral role in the ability of fishers to navigate their environs. Within the range of fishers in British Columbia, ecosystems that support individuals in the Columbian population had significantly higher volumes of coarse woody debris, more overall vegetation cover during winter, and more high-shrub (i.e., 2-10 m tall) cover than did equivalent sites in the Boreal population. As a result, the structural complexity of the forest floor that fishers navigate is significantly different between the two populations. I hypothesise that fishers in the Columbian population developed more compact axial and appendicular skeletons and lighter bodies because the structural complexity of forests in which they occurred was substantially higher than those in which the Boreal population currently exists. If this hypothesis is true, fishers from the Columbian population, with their smaller axial skeletons and feet, would be able to navigate dense, structurally complex forests better than their Boreal population counterparts, who live in much less complex surroundings.
Fishers have considerable diet plasticity (e.g., Kirby et al. 2018) and the observed differences in diet among fishers in British Columbia could simply be the result of different prey densities that occurred in each population. However, the observed differences in morphology, combined with differences in forest structure, may have also affected the ability of fishers in each population to exploit different species of prey and it is possible that the more compact axial skeleton of fishers in the Columbian population were partially the result of adaptations to improve foraging efficiency for mid-sized prey in these dense forests. Further research is needed to determine if differences in prey abundance or foraging efficiency for different prey exist between the 2 populations and to clarify the role of these and other causal agents for the dietary differences observed.
Still other environmental differences between the 2 populations may have contributed to the divergence in morphology observed in this study. Although the ecological niche of fishers throughout their global range includes areas with harsh winters where snow covers the ground for at least part of the year (Powell 1993), the ability of the species to move during winter in an energetically sustainable manner is strongly affected by the condition of the snow upon which they travel. Unlike sympatric predators like Canada lynx Lynx canadensis or wolverines Gulo gulo, deep, soft snow hampers the locomotion of fishers (Raine 1983, Krohn, Zielinski, and Boone 1997) and adaptations to different snow conditions may have contributed to the differences in foot-loading between Columbian and Boreal populations of fishers. Current-day snow conditions are quite different between the 2 populations, with average total annual snowfall being substantially higher in the Boreal White and Black Spruce biogeoclimatic zone in which the Boreal population occurs (x̄ = 245 cm, SD = 57 cm) compared to the biogeoclimatic units in which the Columbian population is most abundant (i.e., x̄ = 159 cm, SD = 47 cm, Interior Douglas-Fir dry-cool subzone; x̄ = 178 cm, SD = 43 cm, Sub-Boreal Pine-Spruce zone; x̄ = 179 cm, SD = 49 cm, Sub-Boreal Spruce dry-warm subzone; data for 1901 to 2019 from MacKenzie et al. 2024). It is unknown when lower foot-loading in fishers from the Boreal population may have arisen, but it is possible that snow conditions during the Pleistocene and Holocene may have exerted selective pressure that resulted in these fishers developing larger feet relative to their body mass. Alternatively, perhaps snow conditions in the Pleistocene refugia that housed the Columbian population were less limiting on fishers, so they could afford to have higher foot-loading than their eastern counterparts.
Heritability of morphological traits
It is possible that the differences in morphology among populations that I observed were simply the result of phenotypic plasticity. That is, the size and shape of fishers in British Columbia may have been the result of variable expression of their morphology among individuals, independent of previous generations, and the result of variable environmental conditions. If phenotypic plasticity was the source of morphometric variation among populations, I would expect that morphometrics would vary considerably over time as environmental conditions changed. I would also expect to see considerable variation within, rather than among, fisher populations that I assessed because carcass samples were collected over an exceptionally large area (i.e., ~220,000 km²) that covered a wide variety of ecosystems and biogeoclimatic zones.
However, I detected differences between populations that were consistent over multiple generations and large geographic areas. Specifically, morphological differences among the carcass samples, which were collected between 1989 and 1993, were very similar to those among the live-capture samples, which were collected between 1996 and 2023. This consistency in morphological differences spanned about 5 generations of fishers, assuming that average generation time is ~3 years. Also, I did not observe excessive variation in morphometric measurements within populations, despite fishers in the Columbian population occurring in at least 4 different biogeoclimatic zones with varying climates. Indeed, variation of many morphometric measurements within this population were similar to that found within the Boreal population, which was dominated by a single biogeoclimatic zone and more stable continental climate.
These observations suggest a genetic basis to the morphological differences among fishers in British Columbia. Morphology is strongly coded in genetic material for many species (e.g., Matthews, Dial, and Lauder 2023) and, although I did not explicitly identify functional genes that produced the morphometrics observed in this study, the conservation of morphological characteristics of each population among generations and across vast areas suggests that morphology of fishers in British Columbia were heritable traits that were passed along to offspring. Future research involving genetic analysis would help confirm whether the morphological and other differences observed in this study are due to adaptations that enhance fitness or are simply expressions of phenological plasticity.
Biogeographical history
Several factors need to be in play for these morphological differences to be the result of adaptive changes: reproductive isolation, differing environments that affect survival and reproduction, and heritable variability in morphology, all of which need to occur for sufficient time for selective filters to operate (e.g., Latta 2010). Within fishers in British Columbia, reproductive isolation sufficient to allow for genetic differentiation has been shown (Weir et al. 2024), forests that currently support fishers in each population are significantly different in their structural attributes and snowfall, and variation in morphology appears to be heritable. However, for adaptive differences in morphology to have developed, the 2 populations of fishers needed to have been separated in sufficiently different environments for an appropriate amount of time for natural selection to have occurred. Recent genetic and paleontological evidence, outlined below, suggests that the Columbian and Boreal populations may have been geographically isolated for at least 20,000 years, which may have contributed to the observed morphological differences forming as the result of adaptative changes within in each population.
Fishers have occurred in North America for at least 7 million years (Samuels and Cavin 2013), but their distribution over this period has varied due to changing geography and climate fluctuations. During the Last Glacial Maximum of the Pleistocene epoch between 13,900 and 30,900 years ago, the current-day range of fishers in Canada was largely covered by massive ice sheets up to 1 km thick (Clague 2017). Numerous paleontological specimens show that fishers in eastern North America persisted in refugia to the south of the Laurentide Ice Sheet and recolonized northward following glacial retreat. Indeed, fishers in the Boreal population of British Columbia likely survived Pleistocene glaciations in the extensive refugia that persisted east of the continental divide and south of the Laurentide Ice Sheet. As glacial ice retreated, these fishers colonized northward into suitable areas (Graham and Graham 1990) that eventually formed the boreal and Carolinian forests of Canada and northeastern USA (Gibilisco 1994). Paleontological evidence confirms that Picea forests had become established in northeastern British Columbia by 10,000 years ago (Hebda 1995) and fishers had colonized this region by 1,470 to 4,270 years ago (Graham and Graham 1994).
The biogeographical history of the Columbian population of fishers, however, is likely substantially different and may involve cryptic refugia. Paleontological specimens of fishers are absent from the Pleistocene record of western North America (i.e., west of the continental divide) and are not documented there until the late Holocene, about 5,000 years ago (Graham and Graham 1994). Initially, this was interpreted as fishers surviving the Pleistocene glaciation only to the south of the Laurentide Ice Sheet, east of the continental divide and colonizing western North America from the east in the late Holocene (Graham and Graham 1994).
However, the timing and formation of new, endemic haplotypes among fishers in western North America is incongruent with a late Holocene colonization from east of the continental divide. Specifically, haplotypes that are endemic to western North American fishers (i.e., 1-BC, 2, 4, 6, 9, 12; Drew et al. 2003) were unlikely to develop within 5,000 years (i.e., ~1,500 generations) if the source of the population was immigration from the east. Knaus et al. (2011) estimated that a single substitution in mitochondrial DNA of fishers occurred every 8,400 years and closer examination of full mitogenome haplotypes from fishers in western North America typically involved 15-20 substitutions from the common ancestor with eastern fishers. Thus, it is highly likely these endemic haplotypes developed in western North America during a period of isolation from their eastern counterparts that spanned the Last Glacial Maximum at 19,500 years bp, and possibly longer. Because of this timeline, fishers in western North America most likely survived the late Pleistocene and early Holocene genetically isolated from eastern counterparts in glacial refugia that existed to the south or west of the Cordilleran Ice Sheet.
If these biogeographical histories for the 2 populations are accurate, the morphological differences between Columbian and Boreal fishers today likely became established because of the different environments in which ancestors to each population occurred during this extensive period of isolation between 4,000 and 30,900 years ago. It is likely that fishers from both populations have only recently begun to share genes at a low level, as evidenced from admixture that occurred in the past but has since diminished with increased barrier effect as the climate changed to current-day conditions (Weir et al. 2024).
Age distribution and sex ratio
The Columbian and Boreal populations of fishers in British Columbia showed consistent patterns of age structure characterized by a prevalence of non-adult age classes with the sex ratio drifting from near equal in younger age classes to being dominated by females in later age categories. Few fishers in either population lived past 4 years of age and both populations developed imbalances in ages between the sexes, with both the Columbian and Boreal populations starting out near 1:1 ratio of females to males, but this ratio generally increased to about 2:1 in older age classes. This pattern is consistent with fisher populations elsewhere, including a survey of 3,566 fisher carcasses collected from trappers in Ontario during the 1970s where, although starting off at 1:1 as juveniles, the ratio of females to males increased to 3:2 by age 2, 7:4 by age 4, and 19:2 by age 6+ (Strickland 1982). Fishers in British Columbia typically do not establish permanent territories until age 2 (Weir, Harestad, and Corbould 2009, Weir et al. 2013), so about 70% of both populations were comprised of non-resident animals that likely do not hold territories. Although this age structure is heavily weighted to young animals, it is consistent with the observations that few transient animals survive to adulthood and the successful acquisition of a home range (Weir, Harestad, and Corbould 2009, Weir et al. 2013, Lofroth et al. 2023).
This age structure suggests that relatively few female fishers survive long enough to produce many offspring. Given that adult female fishers in the Columbian population produce litters once every 2 years of their reproductive life (i.e., denning rate = 0.54; Lofroth et al. 2023), this age-structure data suggests that, on average, only 9% of juvenile female fishers in the Columbian population would be expected to produce 2 litters during their lifetime (i.e., will survive to age 4) and 27% would produce at least 1 litter. By contrast, about 29% of juvenile female fishers in the Boreal population would be predicted, on average, to produce 2 litters during their lifetime (i.e., live to age 3) and 35% would produce at least 1 litter (i.e., denning rate = 0.82; Lofroth et al. 2023).
The dominance of non-adults in both populations was somewhat unexpected based upon survival data of radio-tagged fishers from the 2 populations. Although Lofroth et al. (2023) reported that survival rates for subadult fishers (i.e., <2 yr; 0.64 on average) were significantly lower than that for adults (0.79), this difference alone does not explain the preponderance of non-adult fishers in the age structure of either population. Previous work has suggested that age structure may reflect survivorship only for populations with stable sizes and age structures, which is uncommon among fisher populations (Powell 1994). Furthermore, the abundance of food in the system, which varies over time and space, has been shown to affect juvenile to adult female ratios among harvested fisher populations in Ontario (Greenhorn et al. 2021). Because fishers likely have a numerical response to snowshoe hare populations (e.g., Bowman, Donovan, and Rosatte 2006) and age samples were collected at multiple points in the snowshoe hare population cycle (i.e., fishers’ primary prey in British Columbia; Weir, Harestad, and Wright 2005), it is likely that that the samples simply reflect the population structure at the time of surveys (e.g., 1989-1993 for the carcass sample). The result of drawing samples from numerous points in time may be that the estimated age structure is the average age structure in the system. Alternatively, the sampling may have occurred during times when reproductive productivity was high, resulting in a preponderance of younger age classes in the sample. Additional work that more closely examines prey numbers and population age structure is needed to improve our understanding of the factors that affect age structure within fisher populations in British Columbia.
It is important to note that the age-structure data used in this analysis is likely an imperfect, but adequate, representation of the actual age structure within the population and several factors may have affected its estimation. Sample collection spanned almost 30 years and sampling periods for carcass and live-capture collections captured very different periods in the sustainability of either population as well, which likely contributed to observed differences among the data sets. The age structure of samples from live captures of fishers is likely the best reflection of the true age structure within the populations. Unlike fur-trapping, where kill-traps are placed for a period then moved (often depending on whether animals were killed or not), live-capturing fishers for radio-tagging is often much more intensive (i.e., many livetraps per territory) and of greater effort (i.e., many trap-nights per livetrap) as the desired outcome is to have all fishers in a study area captured and radio-tagged (e.g., 9,724 trap-nights in 1,930 km², Weir and Corbould 2006; 4,003 trap-nights in 950 km², Weir, Lofroth, and Phinney 2011). As such, age structure determined from live-capture is likely less biased towards detecting only animals with higher susceptibility to capture, such as juveniles and adult males (Strickland and Douglas 1981, Krohn, Arthur, and Paragi 1994, Greenhorn et al. 2021), as the intensity of sampling is much higher during live-capture programs than in standard fur harvest kill-trapping.
Reproduction
Corpora lutea counts were not significantly different between fisher populations in British Columbia in the early 1990s but were lower than that recorded elsewhere. In Ontario, the average count of corpora lutea among fisher carcasses collected during the 1970s was 3.16 per adult female (Strickland 1982), which was substantially higher than the 2.35 observed in the Columbian and 2.42 in the Boreal populations of British Columbia. The rate at which adult females were non-reproductive (i.e., no corpora lutea) in both British Columbian populations (22% in Columbian; 25% in Boreal) were substantially higher than that found in Ontario (~5%, Strickland 1982). Interestingly, the proportion of carcasses with visible fat deposits was similar among populations and between British Columbia and Ontario (90% in Ontario, Strickland 1982; 89% in Columbian population, 86% in Boreal population), so this discrepancy may not be related to food supply. It is unclear whether an innate difference in reproductive output exists between British Columbia and Ontario, or if other factors caused this difference. Because corpora lutea counts are only useful as a maximal estimate of reproductive output, it is also unclear as to whether differences in corpora lutea counts resulted in real differences in reproductive output. Furthermore, these corpora lutea counts were obtained from fishers collected in 1989-1993, when population productivity was likely considerably higher than currently found. More recent estimates of kit productivity show significantly lower reproductive output among radio-tagged fishers in the Columbian population than that in the Boreal population (Lofroth et al. 2023).
Columbian subspecies P. p. columbiana
Morphological differences that may be adaptations to their local environments (this study), recent genetic evidence (Drew et al. 2003, Schwartz 2007, Knaus et al. 2011, Weir et al. 2024), and different biogeographical histories provide consistent evidence to support the hypothesis that fishers in the Columbian population may constitute a valid subspecies. Several factors likely contributed to the earlier debate about the legitimacy of subspecies in fishers.
Previous assessments of subspecies in fishers, such as the extensive survey by Hagmeier (1959), were largely limited to skull morphometrics, but my study found skull morphometry to be relatively invariant compared to other features. As a result, it is possible that these studies failed to detect subspeciation, not because it was not present, but because researchers chose to examine relatively invariant attributes. As case in point, Hagmeier (1958) failed to detect differentiation or subspeciation among North American martens Martes using the same skull-morphometric approach as he used on fishers. Later genetic and morphometric evidence, however, showed that his sample of martens was actually comprised of 2 completely different species (American martens M. americana and Pacific martens M. caurina; Dawson and Cook 2012) and several subspecies (e.g., M. a. atrata, Kyle and Strobeck 2003; M. c. humboltensis, Schwartz et al. 2020). It is possible that previous assessments of subspeciation in fishers would have different conclusions had researchers had access to the entire skeleton of fishers. The lack of variation in skull morphometrics relative to other morphometric features may be the result of selective pressure for conservation of skull size and shape to allow for continued exploitation of specific prey items (e.g., Holmes and Powell 1994). As such, assessing skull morphometry may not be an adequate method by which to evaluate subspeciation in fishers, martens, and possibly other Guloninae. In addition to the invariant nature of skull morphometry, many specimens used in previous assessments appear to have been mistakenly assigned to incorrect subspecies. This is particularly true in the assessments of Goldman (1935) and Hagmeier (1959), both of whom considered specimens collected as far east as Manitoba to be P.p. columbiana, despite fishers from this region likely belonging to the P.p. pennanti subspecies. A more definitive analysis of the validity of the subspecies of fishers, however, is best conducted using genetic analysis (e.g., Schwartz et al. 2020).
Although differences in morphology between the 2 populations were consistent with heritable traits that could be adaptive to the environments in which each population occurred, I did not explicitly examine changes in fitness in response to environmental variation within each population. Possible evidence that these differences are adaptive traits that affect fitness, however, may be found in the common-garden experiment-like translocation of 149 fishers from both the Columbian and northern Alberta (i.e., boreal) populations into the Cascade Mountain Range of Washington State (Lewis et al. 2022). Ecosystems in the Cascade Mountain Range are floristically and structurally similar to those found in the southern portion of the Columbian population (i.e., dominated by Douglas-fir forests and having relatively high structural complexity; Spies et al. 1988). Fishers translocated from the Columbian population had substantially higher survival rates (~0.76) than did those translocated from the boreal forests of northern Alberta (~0.40). The higher survival rate of fishers from the Columbian population may have occurred because their morphological and behavioural adaptations were better suited to this environment than those of fishers from the boreal forest, although habitat supply at release sites may have affected survival somewhat (Lewis et al. 2022).
Implications for population recovery
Fishers in the Columbian population seem to have developed morphological and possibly behavioural adaptations to their environment not expressed within the Boreal population. Fishers in the Columbian population may have had a significant period of isolation that likely generated an evolutionary history with localized adaptations that are heritable and unlikely to be practically reconstituted if this population were lost because individuals from other populations will not have the same heritable adaptive traits or hallmarks of the same evolutionary history as those they are replacing. Specifically, I predict that if fishers from the Boreal population were moved to the central interior of British Columbia, they would not survive and reproduce as well as fishers from the Columbian population (as was observed empirically with translocations into Washington State) because their larger axial skeleton and larger feet would make navigating the more structurally complex forests of the interior of British Columbia more difficult and would put them at a competitive disadvantage to other sympatric predators. Additional studies, such as whole mitogenome and other genetic analyses to evaluate the heritability of observed phenotypic traits, behavioural fitness research, and a closer examination of putative biogeographical histories, are needed to examine these untested hypotheses.
Acknowledgments
Carcass collections were coordinated by R. Wright and others at the BC Ministry of Environment and A. Harestad at Simon Fraser University. Thanks to L. Davis, J. Lewis, and S. Frey for contributing morphometric and age data on live-captured fishers from their respective studies and J. Gorrell for assistance interpreting genetic data. This manuscript benefited from the review of H. Davis, J. Burgar and 5 anonymous reviewers.
Author Contributions
Richard Weir: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing
Data Availability
Portions of data are available upon request.
Supplemental Information
Supplemental Information can be found here.
Transparent Peer Review
Results from the Transparent Peer Review can be found here.
Recommended Citation
Weir, R.D. (2025). Differentiation and adaptation among fishers in Western Canada: Evidence for validity of Pekania pennanti columbiana subspecies. Stacks Journal: 25002. https://doi.org/10.60102/stacks-25002
References
Bowman, Jeff, Dennis Donovan, and Richard C. Rosatte. 2006. “Numerical response of fishers to synchronous prey dynamics.” Journal of Mammalogy 87 (3): 480-484. https://doi.org/10.1644/05-MAMM-A-202R2.1.
British Columbia Ministry of Forests and Range, and British Columbia Ministry of Environment. 2010. Field manual for describing terrestrial ecosystems. Province of British Columbia (Victoria, British Columbia, Canada). https://www2.gov.bc.ca/assets/gov/environment/plants-animals-and-ecosystems/conservation-data-centre/field_manual_describing_terrestrial_ecosystems_2nd.pdf.
Clague, John J. 2017. “Deglaciation of the Cordillera of Western Canada at the end of the Pleistocene.” Cuadernos de Investigación Geográfica 43 (2): 449-466. https://doi.org/10.18172/cig.3232.
Davis, Larry R. 2009. “Denning ecology and habitat use by fisher (Martes pennanti) in pine dominated ecosystems of the Chilcotin Plateau.” Thesis, Simon Fraser University, Burnaby, British Columbia, Canada. 109 pages.
Dawson, Natalie G., and Joseph A. Cook. 2012. “Behind the genes: Diversification of North American martens (Martes americana and M. caurina).” In Biology and Conservation of Martens, Sables, and Fishers: A New Synthesis, edited by Keith B. Aubrey, William J. Zielinski, Martin G. Raphael, Gilbert Proulx and Steven W. Buskirk, 23-38. Ithaca, New York, USA: Cornell University Press.
Douglas, Carman W., and Marjorie A. Strickland. 1987. “Fisher.” In Wild furbearer management and conservation in North America, edited by Milan Novak, James A. Baker, Martyn E. Obbard and Bruce Malloch, 511-529. North Bay, Ontario, Canada: Ontario Trappers Association.
Drew, R. E., J. G. Hallett, K. B. Aubry, K. W. Cullings, S. M. Koepf, and W. J. Zielinski. 2003. “Conservation genetics of the fisher (Martes pennanti) based on mitochondrial DNA sequencing.” Molecular Ecology 12(1): 51-62. https://doi.org/10.1046/j.1365-294X.2003.01715.x.
Gibilisco, Charles J. 1994. “Distributional dynamics of modern Martes in North America.” In Martens, sables, and fishers: Biology and conservation, edited by Steven W. Buskirk, Alton S. Harestad, Martin G. Raphael and Roger A. Powell, 59-71. Ithaca, New York, USA: Cornell University Press.
Goldman, F. A. 1935. “New American mustelids of the genera Martes, Gulo, and Lutra.” Proceedings Biological Society of Washington 48: 175-186. https://www.biodiversitylibrary.org/page/34563831.
Graham, Mary Ann, and Russell W. Graham. 1990. “Holocene records of Martes pennanti and Martes americana in Whiteside County, northwestern Illinois.” American Midland Naturalist 124 (1): 81-92. https://doi.org/10.2307/2426081.
Graham, Russell W., and Mary Ann Graham. 1994. “Late Quaternary distribution of Martes in North America.” In Martens, sables, and fishers: Biology and conservation, edited by Steven W. Buskirk, Alton S. Harestad, Martin G. Raphael and Roger A. Powell, 26-58. Ithaca, New York, USA: Cornell University Press.
Greenhorn, Janet E., Jeff Bowman, Simon T. Denomme-Brown, and Danielle M. Ethier. 2021. “Bottom–up trophic effects on fisher Pekania pennanti harvest age structure: associations with mast, voles and owls.” Wildlife Biology 2021 (4): wlb.00873. https://doi.org/10.2981/wlb.00873.
Grinnell, Joseph, Joseph S. Dixon, and Jean M. Linsdale. 1937. “Fisher.” In Furbearing mammals of California: Their natural history, systematic status, and relations to man, 211-230. Berkeley, California, USA: University of California Press.
Hagmeier, Edwin M. 1958. “Inapplicability of the Subspecies Concept to North American Marten.” Systematic Zoology 7 (1): 1-7. https://doi.org/10.2307/2411472.
Hagmeier, Edwin M.. 1959. “A re-evaluation of the subspecies of fisher.” Canadian Field Naturalist 73:185-197. https://www.biodiversitylibrary.org/partpdf/341819.
Hebda, Richard. 1995. “British Columbia Vegetation and Climate History with Focus on 6 ka BP.” Géographie physique et Quaternaire 49 (1): 55-79. https://doi.org/10.7202/033030ar.
Holmes, Thor, and Roger A. Powell. 1994. “Morphology, ecology, and the evolution of sexual dimorphism in North American Martes.” In Martens, sables, and fishers: Biology and conservation, edited by Steven W. Buskirk, Alton S. Harestad, Martin G. Raphael and Roger A. Powell, 72-84. Ithaca, New York, USA: Cornell University Press.
Kirby, Rebecca, Carissa Freeh, Jonathan H. Gilbert, John F. Olson, and Jonathan N. Pauli. 2018. “Poor body condition and diet diversity in a harvested population of fishers.” Wildlife Biology 2018 (1): wlb.00334. https://doi.org/10.2981/wlb.00334.
Knaus, Brian J., Richard Cronn, Aaron Liston, Kristine Pilgrim, and Michael K. Schwartz. 2011. “Mitochondrial genome sequences illuminate maternal lineages of conservation concern in a rare carnivore.” BMC ecology 11 (1): 10. https://doi.org/10.1186/1472-6785-11-10.
Krohn, William B., Stephen M. Arthur, and Thomas F. Paragi. 1994. “Mortality and vulnerability of a heavily-trapped fisher population.” In Martens, sables, and fishers: Biology and conservation, edited by Steven W. Buskirk, Alton S. Harestad, Martin G. Raphael and Roger A. Powell, 137-146. Ithaca, New York, USA: Cornell University Press.
Krohn, William B., William J. Zielinski, and Randall B. Boone. 1997. “Relations among fishers, snow, and martens in California: Results from small-scale spatial comparisons.” In Martes: Taxonomy, ecology, techniques, and management, edited by Gilbert Proulx, Harold N. Bryant and Paul M. Woodward, 211-232. Edmonton, Alberta, Canada: Provincial Museum of Alberta.
Kyle, Christopher J., and Curtis Strobeck. 2003. “Genetic homogeneity of Canadian mainland marten populations underscores the distinctiveness of Newfoundland pine martens (Martes americana atrata).” Canadian Journal of Zoology 81 (1): 57–66. https://doi.org/10.1139/Z02-223.
Latta, Robert G. 2010. “Natural Selection, Variation, Adaptation, and Evolution: A Primer of Interrelated Concepts.” International Journal of Plant Sciences 171 (9): 930-944. https://doi.org/10.1086/656220.
Lewis, Jeffrey C. 2014. “Post-Release Movements, Survival, and Resource Selection of Fishers (Pekania pennanti) Translocated to the Olympic Peninsula of Washington.” Dissertation, University of Washington, Seattle, USA.
Lewis, Jeffrey C., Jason I. Ransom, Tara Chestnut, David O. Werntz, D. Whiteside, J. L. Postigo, and A. Moehrenschlager. 2022. Cascades fisher reintroduction project: Final project report. National Parks Service. Fort Collins, Colorado, USA. https://conservationnw.org/wpcontent/uploads/2022/07/2022_Lewis_et_al_Cascades_fisher_final_report.pdf.
Lofroth, Eric C., Richard D. Weir, Larry R. Davis, and Ingebjorg-Jean Hansen. 2023. “A tale of two populations: vital rates of fishers in British Columbia, Canada.” Journal of Wildlife Management 87:e22315. https://doi.org/10.1002/jwmg.22315.
MacKenzie, William H., David K. Daust, H Griesbauer, E. Matsuzaki, Vanessa Foord, and C. Mahony. 2024. “Historic and future projected climate summaries of Biogeoclimatic units of British Columbia and adjacent jurisdictions.” Ministry of Forests (Victoria, British Columbia, Canada). Accessed 3 February 2024. https://thebeczone.ca/shiny/bybecmap/.
MacKenzie, William H., and Del V. Meidinger. 2018. “The Biogeoclimatic Ecosystem Classification Approach: an ecological framework for vegetation classification.” Phytocoenologia 48 (2): 203-213. https://doi.org/10.1127/phyto/2017/0160.
MacKinnon, A, D Meidinger, and K Klinka. 1992. “Use of the biogeoclimatic ecosystem classification system in British Columbia.” The Forestry Chronicle 68 (1): 100-120. https://doi.org/10.5558/tfc68100-1.
Matthews, David G, Terry R Dial, and George V Lauder. 2023. “Genes, Morphology, Performance, and Fitness: Quantifying Organismal Performance to Understand Adaptive Evolution.” Integrative and Comparative Biology 63 (3): 843-859. https://doi.org/10.1093/icb/icad096.
Oksanen, J., G. Simpson, F. Blanchet, R. Kindt, P. Legendre, P. Minchin, R. O’Hara, P. Solymos, M. Stevens, E. Szoecs, H. Wagner, M. Barbour, M. Bedward, B. Bolker, D. Borcard, G. Carvalho, M. Chirico, M. De Caceres, S. Durand, H. Evangelista, R. FitzJohn, M. Friendly, B. Furneaux, G. Hannigan, M. Hill, L. Lahti, D. McGlinn, M. Ouellette, E. Ribeiro Cunha, T. Smith, A. Stier, C. Ter Braak, and J. Weedon. 2024. vegan: Community Ecology Package 2.6.8. https://CRAN.R-project.org/package=vegan.
Pojar, Jim, K. Klinka, and D. V. Meidinger. 1987. “Biogeoclimatic ecosystem classification in British Columbia.” Forest Ecology and Management 22: 119-154. https://doi.org/10.1016/0378-1127(87)90100-9.
Poole, Kim G., Gary M. Matson, Marjorie A. Strickland, Audrey J. Magoun, Ron P. Graf, and Linda M. Dix. 1994. “Age and sex determination for American martens and fishers.” In Martens, sables, and fishers: Biology and conservation, edited by Steven W. Buskirk, Alton S. Harestad, Martin G. Raphael and Roger A. Powell, 204-224. Ithaca, New York, USA: Cornell University Press.
Powell, Roger A. 1993. The fisher: Life history, ecology, and behavior. Second edition. Minneapolis, Minnesota, USA: University of Minnesota Press. Powell, Roger A. 1994. “Structure and spacing of Martes populations.” In Martens, sables, and fishers: Biology and conservation, edited by Steven W. Buskirk, Alton S. Harestad, Martin G. Raphael and Roger A. Powell, 101-121. Ithaca, New York, USA: Cornell University Press.
Raine, R. Michael. 1983. “Winter habitat use and responses to snow cover of fisher (Martes pennanti) and marten (Martes americana) in southeastern Manitoba.” Canadian Journal of Zoology 61: 25-34. https://doi.org/10.1139/z83-002.
Resource Inventory Committee. 1998. Live animal capture and handling guidelines for wild mammals, birds, amphibians, and reptiles. Province of British Columbia (Victoria, British Columbia, Canada). https://www2.gov.bc.ca/assets/gov/environment/natural-resource-stewardship/nr-laws-policy/risc/capt.pdf
Rhoads, Samuel N. 1898. “Contributions to a revision of the North American beavers, otters and fishers.” Transactions of the American Philosophical Society 19 (3): 417-439. https://doi.org/10.2307/1005498.
Samuels, Joshua X., and Jennifer Cavin. 2013. “The earliest known fisher (Mustelidae), a new species from the Rattlesnake Formation of Oregon.” Journal of Vertebrate Paleontology 33 (2): 448-454. https://doi.org/10.1080/02724634.2013.722155.
Schwartz, Michael K., Ashley D. Walters, Kristine L. Pilgrim, Katie M. Moriarty, Keith M. Slauson, William J. Zielinski, Keith B. Aubry, Benjamin N. Sacks, Katherine E. Zarn, Cate B. Quinn, and Michael K. Young. 2020. “Pliocene–Early Pleistocene Geological Events Structure Pacific Martens (Martes caurina).” Journal of Heredity 111 (2): 169-181. https://doi.org/10.1093/jhered/esaa005.
Schwartz, Michael K. 2007. “Ancient DNA confirms native Rocky Mountain fisher (Martes pennanti) avoided early 20th century extinction.” Journal of Mammalogy 88 (4): 921-925. https://doi.org/10.1644/06-MAMM-A-217R1.1.
Simpson, Keith, T. K. Simpson, Lauren Simpson, Lorraine Andrusiak, Shawn Hilton, Amanda Kellner, Richard Klafki, Ingebjorg-Jean Mattson, and A. Creagh. 2013. Part 7 Mammals. Terrestrial Vegetation and Wildlife Report. Site C Clean Energy Project. Report prepared for BC Hydro (Vancouver, British Columbia, Canada). http://www.ceaa-acee.gc.ca/050/documents_staticpost/63919/85328/Vol2_Appendix_R-7-Mammals.pdf
Spies, Thomas A., Jerry F. Franklin, and Ted B. Thomas. 1988. “Coarse Woody Debris in Douglas-Fir Forests of Western Oregon and Washington.” Ecology 69 (6): 1689-1702. https://doi.org/10.2307/1941147.
Strickland, Marjorie A. 1982. Fisher and marten study 1979-80 and 1980-81 Algonquin region. Ontario Ministry of Natural Resources (Owen Sound, Ontario, Canada).
Strickland, Marjorie A., and Carman W. Douglas. 1981. “The status of fisher in North America and its management in southern Ontario.” Proceedings of the worldwide furbearer conference, Baltimore, Maryland, USA.
Tabachnick, Barbara G, and Linda S. Fidell. 2019. Using Multivariate Statistics. New York, New York, USA: Peason Education Inc. Seventh edition.
Weir, Richard D. 1995. “Diet, spatial organization, and habitat relationships of fishers in south-central British Columbia.” Thesis, Simon Fraser University, Burnaby, British Columbia, Canada. 135 pages.
Weir, Richard D., and Fraser B. Corbould. 2006. “Density of fishers in the Sub-boreal Spruce biogeoclimatic zone of British Columbia.” Northwestern Naturalist 87 (2): 118-127. https://doi.org/10.1898/1051-1733(2006)87[118:DOFITS]2.0.CO;2.
Weir, Richard D., and Fraser B. Corbould. 2008. Ecology of fishers in the sub-boreal forests of north-central British Columbia. Final Report. Peace/Williston Fish and Wildlife Compensation Program (Prince George, British Columbia, Canada). http://a100.gov.bc.ca/pub/siwe/details.do?id=4806.
Weir, Richard D., and Alton S. Harestad. 2003. “Scale-dependent habitat selectivity by fishers in south-central British Columbia.” Journal of Wildlife Management 67 (1): 73-82. https://doi.org/10.2307/3803063.
Weir, Richard D., Alton S. Harestad, and Fraser B. Corbould. 2009. “Home ranges and spatial organization of fishers in central British Columbia.” Canadian Field Naturalist 123: 126-132. https://doi.org/10.22621/cfn.v123i2.690.
Weir, Richard D., Alton S. Harestad, and Randy C. Wright. 2005. “Winter diet of fishers in British Columbia.” Northwestern Naturalist 86 (1): 12-19. https://doi.org/http://www.jstor.org/stable/4095775.
Weir, Richard D., Eric C. Lofroth, and Mark Phinney. 2011. “Density of fishers in boreal mixedwood forests of northeastern British Columbia.” Northwestern Naturalist 92 (1): 65-69. https://doi.org/10.1898/10-15.1.
Weir, Richard D., Eric C. Lofroth, Mark Phinney, and Leanne R. Harris. 2013. “Spatial and genetic relationships of fishers in boreal mixed-wood forests of northeastern British Columbia.” Northwest Science 87 (2):114-125. https://doi.org/10.3955/046.087.0204.
Weir, Richard D., Mark Phinney, and Eric C. Lofroth. 2012. “Big, sick, and rotting: Why tree size, disease, and decay are important to fisher reproductive habitat.” Forest Ecology and Management 265 (1): 230-240. https://doi.org/10.1016/j.foreco.2011.10.043.
Weir, Richard D., Andrew A. Rankin, Lacy Robinson, Kristine L. Pilgrim, Michael K. Schwartz, and Michael K. Lucid. 2024. “Genetic structuring of fishers in British Columbia, Canada: Implications for population conservation and management.” Journal of Mammalogy 105 (3): 465-480. https://doi.org/10.1093/jmammal/gyae007.
Zielinski, William J., and Richard L. Truex. 1995. “Distinguishing tracks of marten and fisher at track-plate stations.” Journal of Wildlife Management 59 (3): 571-579. https://doi.org/10.2307/3802465.
Accepted by 5 of 5 reviewers
Open Access
Peer-Reviewed
Creative Commons
Submitted: 25 April 2024
Accepted: 24 February 2025
Published: 21 April 2025
Funding Information: Abitibi Consolidated, British Columbia Hydro and Power Authority, British Columbia Ministry of Environment, British Columbia Ministry of Forests Forest Science Section, British Columbia Timber Sales, British Columbia Trappers Association, EnCana Corporation, Forest Renewal British Columbia, Forest Sciences Program of the British Columbia Forest Investment Account, Habitat Conservation Fund, Habitat Conservation Trust Foundation, Louisiana-Pacific Canada, Peace/Williston Fish and Wildlife Compensation Program, Science Council of British Columbia, Simon Fraser University, Slocan Group, Tembec, Tolko Industries, Tsi Del Del Enterprises, Washington Department of Fish and Wildlife, West Fraser Mills, and Yun Ka Whu’ten Holdings.
Conflicts of Interest: The author declares no conflicts of interest.
© 2025 Weir. Stacks Journal